On the sea, the material is severely tested by seawater for the careen and salt air for topsides. Their slow and gradual chemical action on metal cause corrosion.
1)Voltaic pile working
Invented by Alessandro Volta, it consisted of a stack of alternating discs of zinc and copper separated by paperboard soaked in salt water.
The negative terminal is a zinc disc(anode)and the positive terminal is a copper disk(cathode). The electrolyte in this case is the impregnated salt water paperboard. The voltage creates an electrons motion, zinc will be corroded(it is always anode which will be corroded)and electrons lost by zinc will go on cathode(here, copper). It is a redox reaction in which zinc is the reductant and copper oxidant . To summarize a zinc atom loses two electrons migrated on copper, next to the cathode these electrons , through a reduction reaction will create dihydrogen(2H20 and 2é give 2OH- and H2).
Roughly the same story in seawater. By immersing a copper plate and a zinc plate close each other, zinc corrodes itself. Here sea water acts as the electrolyte. The phenomenon is accelerated by voltage between those two plates or by increasing the temperature of water. This is why we must focus our attention on electric power losses on aluminum boats. By taking two different kind of metals, for example aluminum and bronze, we see the same principle with the aluminum which corrodes itself(to install a bronze valve on an aluminum hull…be careful!).
2) Strong / weak metals
the metal role(cathode or anode)isn't fixed, it depends on the metal nature which it is associated by direct or indirect link(salt air, salt water). Here is a list of metals from the more resistant to the weakest against galvanic corrosion:
Stainless steel, Nickel, Bronze, Bronze / aluminum alloy, Copper, brass, Lead, cast iron, Steel, Aluminium alloy, Aluminium and Zinc at the end.
(Notice: Stainless steel A4 type is the one to use in marine environment)
With that you can foresee in the marine environment “who will eat who”:
For example by combining Nickel with Brass, the anode is the Brass. By combining the cast iron with Steel, the anode will be steel. The more a large gap between two metals is in this list and the more galvanic effects will be important(Inox and Zinc=Bingo!).
3)Precautions
For two metal things in contact:
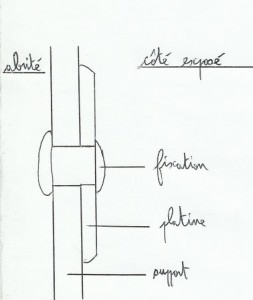
For example, we take an aluminium mast("support")where we want to fix a stainless steel plate(example the spi pole ring). connections must be of the same metal as the object set(by a stainless steel rivet for example), and the plate must always be isolated from the support by an elastomeric sealant( sika type). Otherwise, welcome damages! Connexion(rivet, screw)must also be isolated from the support(elastomeric sealant for rivet and grease for screw ). Always take care to trim the drilled holes and clean the contact areas before setting, you have to avoid stainless steel turnings under the plate, causing corrosion by puncture(also avoid scratches on parts).
For two parts of the same metal, it should also be isolated by a mastic. If the plate is not chamfered, you must do a chamfer in elastomeric sealant around the plate to avoid water entrances(differential aeration corrosion).
4)Anodes
From what we have seen, metal parts on a boat are subject to electrolytic phenomena, So touched by progressive destruction. To avoid this kind of fuses are used on board, it's anodes. It will be destroyed instead of the metal parts of the boat. These anodes must have to be changed regularly. They are made of either aluminum / Zinc alloy or Aluminum(weakest metals listed just above). From what we have seen in the number 2 paragraph, aluminum anodes last longer in time, they also provide better protection. Anodes protect the hull, propeller, Propeller shaft, the inboard engine. On freshwater anodes used are in aluminium, On the sea we rather use alu/zinc alloy anodes.