After what we have seen before, if we resume it we know that:
1) Seebeck effect is able to generate electricity from a thermal differential. Thanks to an electronic component called peltier module, we convert thermal energy into electricity energy. According to a file from insa findable on the net (Rapport_P6-3_2008_32 (1).pdf), If on cold side of the module temperature used is 38 ° C and on the hot face 90 °C (in fact with a differential of 50 ° C.) then voltage made will be 1.161 Volts and power we get 0.674 watts roughly. The yield of this effect is still very low ( 2.4 %). module use is inadvisable for temperatures above 100 °C (it could burn and damage it) According to the provider, but knowing that these modules are made of bismuth tellure (melting at 573 ° C), of copper (melting at 1085 ° C) and ceramics (melting beyond 2000 ° C) perhaps we could push heating before breaking…
2) To have an effective thermal generator, it must provide a regular electric current, so thermal differential must be stabilised. the problem is that after a moment cold face tends to warm up, and get balanced with hot face because it can't exhaust all heat cumulated so thermal differential decrease, and at the end power generated falls.
3) for getting regular energy, we have maybe to consider thermal energy going through the module like a river flowing. If river's flow is steady, then the mill (our module) will provide mechanical energy (electric) steadily. thermal energy must have a regular flow so.
So how to improve cold side to get a regular thermal flow ?
Part 1: some thermodynamic.
Thermodynamics is a branch of science that studies energies exchanges between systems, energy is transfered with heat and work (the 2 they are expressed in joules both); now we will be focused on heat. heat is seen like an energy trade towards systems in a chaotic form, useless (The more heat transfered is strong, the more atoms which convey this heat vibes), and temperature is rather used to talk about the system's state (see the book “Thermodynamic” R. Taillet for example).
Heat exchanges are often assimilated to temperature changes from systems included in those exchanges. this heat is defined by the formula:
Q=CΔT (Q is heat given to the system expressed in joules, C the calorific capacity of the system expressed in joules per kelvin, and ΔT its temperature variation). if we keep on eye on this formula we easily notice that the more calorific capacity of a system is high, the more this system is able to’ store heat (for the same temperature differential between 2 different systems, one having the largest heat capacity will, will capture more heat). the more amount of material is important, the more calorific capacity is important too (we will come back to it soon).
The most of time, in the main ways a dense element is a better heat conductor and has a high volumic thermal capacity, and on an another way for a lightweight element it's not (more insulating, low heat capacity). Metals are good conductors of heat.
Finally, we should consider a thing (with metal ?) dense with lots of material to make an efficient cold face. The sea should be this thing, even able to be an ideal thermostat for our generator (the sea is able to recover the heat produced by the battery without temperature changes, like a thermostat).
Part 2: Solar thermal battery.
1) battery test 1
For the first test i have made a kind of big rectangular box, isolated inside and heated by Fresnel lenses. Lightrays were focused inside on heat collectors made in aluminium and painted in black (paint used is for ovens and fireplaces and can stand 1000°C) and isolated with plaster.
These heat collectors were in contact with Peltier's modules, and modules were in contact with aluminium parts fixed behind the box used like heat dissipaters, heat sinks. This battery included 20 Peltier's modules connected as follows: 10 time series 2 then 2 series of 10 modules in parallel. On a collector we had 2 modules one above the other.
Finally after a test in october during a shiny normandy day this first battery managed to deliver 4.5 V steadily for 20 minutes, but with not a high power (there were roughly 0.5 watts, not enough for a Jean Michel Jarre concert). then i have tried to limitate thermal losses by diving heat collectors in epoxy resin (the idea was to let sunrays going on collectors, but to stop outgoing heat), but Fresnel lenses have too focused rays (i must have changed lenses position a little) and in fact a part has burnt… It works not very good now, except as a bedside table (heavy and ugly) if you possess a strange taste.
2) battery test 2
this time for this new model i have used 6 Fresnel lenses, 6 peltier's modules, a rectangular section of aluminium 1 meter long, 2 copper tubes with a diameter of 18 mm, a wooden board to make the lenses support, and a copper roller. for links i have used serflex and mastic. and of course i have used a hose too :
to make it it's really easy, on the aluminium section i have stuck peltier's modules (only on the corners to avoid a bad heat transfer), and on modules i have stuck copper parts painted in black, useful to grab the light focused with lenses (it's fireplace painting). Then in the aluminum section i have fixed 2 copper tubes, and i have shut 2 aluminium section's holes with mastic. then i have fixed the lenses support with an eye on a light focus not too concentrated (otherwise it makes the paint burning).
after this i have fixed 2 radiators with the copper's roller. each radiator is linked to a copper's tube.
it's working easily: the lense focuses sunrays on the copper part painted in black so it heats, heating also the module . the peltier's module is cooled thanks to the rectangular's aluminium section filled up with water and including copper tubes linked to radiators, also filled up with water. in fact the alu section is used like a heat exchanger between modules and radiators.
Résults:
This battery has been tested between 11h and 13h on 22 august. the circuit wasn't so watertight so i have added up 3 water bottles during 2 hours in radiators. i have used tap water at 17°C roughly.
11h10: 7.7 Volt / 200mA over
11h20: 7.8 Volt / 200mA over
11h30: 7.4 Volt / 200mA over
11h40 (first addition of water): 7.8 Volt / 200mA over
11h50: 7.9 Volt / 200mA over
12h00: 7.7 Volt / 200mA over
12h10: 7.4 volts / 250 mA
12h20 (second addition of water): 8.1 volts/300mA
12h30: 8 volts / 280 mA
12h40: 7.7 Volt / 270 mA
12h50: 7.7 volts / 260 mA
So this battery provides between 1.85 and 2.4 watts of power. during the test i had to move the pile a little every 10 minutes to have it in line with the sun. how far is it efficient ?
In the book “astronomical and mathematical essential for all ” (A. Moatti), the author tells us that the thermal sun flow grabbed on the floor is around 490 Watts per m2. This value varies with the position of the sun relative to our zenith, albédo, cloud cover… so we will just use this number to simplify. Lenses used have a size of 24.5/17 cm so in surface 416.5 cm2. i have used 6 lenses so it's 2499 cm2 then 0.25 m2. finally our generator can pick up from the sun 122.5 watts. So its efficiency is located approximately between 1.5 and 1.9 %. i should try an another test in winter this time to compare data, after improving the radiator's sealing. sure it's possible to win some efficiency again.
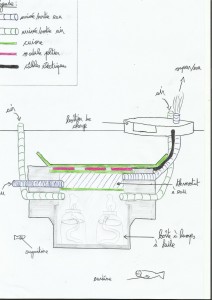
Part 3: thermal oil pile.
As we have seen at the beginning, the sea can perhaps do a great thermostat for thermal battery (So very useful to conduct heat), so currently i am making a diving generator working with oil burning (frying, colza, olive… in fact oil). oil lamps would heat copper, and copper in a second step would heat modules. The cool side would be just a copper board linked with the sea. Here's a diagram of the project:
For the moment i am just making the hot side but it brings several problems:
– for oil combustion we need oxygen, or if the hot part is immersed, it will be difficult to bring a sufficient oxygen volume .
– If the battery is submerged, then it must not have a big volume because of archimede (Archimede's principles) to keep it under water.
– if the pile isn't emmerged but is floating on the water, then we will have to isolate the hot side very well.
– We'll have to adjust the hot part: finding the good balance between heat/oil lamps distance to avoid modules burnt, or doing a thermostat (see the little draw) for limiting the heating temperature of the modules (in the case of the draw it's 100°C).
– heated well, copper changes its shape a little and it could damage modules fixed on it.
These are small pictures of were i am currently:
The box contains 9 Blédina cans (apple/mago taste, my favorites) acting as oil lamps. this box is storable in a copper and wooden cover with 8 holes 1 cm in diameter able to get space for oxygen. it's not efficient enough (only the central oil lamp is still working); if I just let this lamp after 5 minutes it heats the copper board until 50 ° C.
see you soon for the other part 4 I'll try not to take 2 years to do it this time !

















I allow myself a remark or two coming from my interest in peltiers but without too much practice,,,,to insulate the submerged cold part can be with a formwork on the edges waterproof and filled with a balloon,you will only have to increase the pressure and besides you can use a manometer to increase the pressure according to the swell and the weight,,,,and for the oil I just saw your idea but I know the oil lamps very often having to help me from EDF,,your wick will not last long and in addition the oil level will drop,,,so put your candle on the float,,,square is not bad because the edge being occupied by the air chamber you have all the middle left and close the top with peltiers but leaving a few holes at the base a little above the waterline for the oxygen of the candle and a little upwards to avoid the drafts of the wind,,,,I saw that you have a lot of candles and it will require too much maintenance so the middle of your square can be filled with oil and you can make a larger wick or even circular and in x ,,,,This is just what comes to me I admit that I have not read everything, wanting to remain integrated for the moment and not to spoil the experiment.,,,,and first of all who is this shovelman to enlighten my nights?,,,,answer me on my email for the advancement of your project if you feel like it,,,cordially,,,brice
Hello thanks for the advice ! Yes it might be too many candles…I finish (finally) the project to see what happens and I'll keep you posted. Bonne journée.